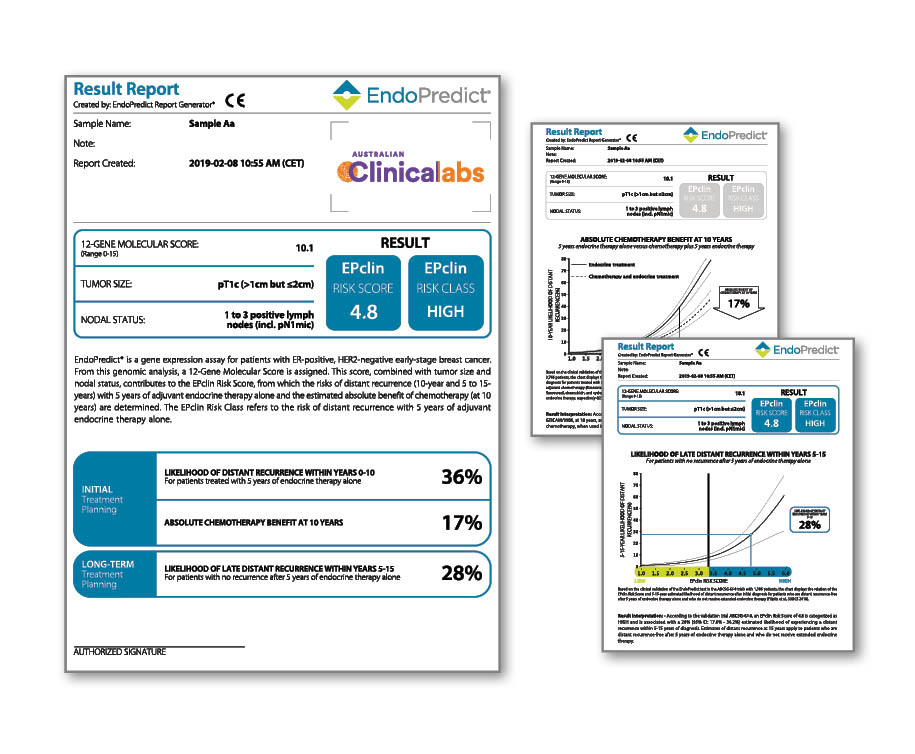
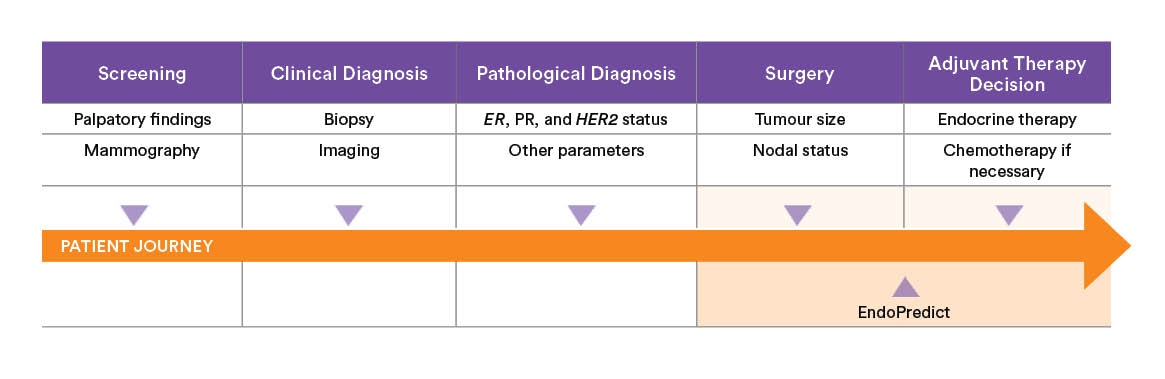
An advanced gene expression test for predicting the risk of distant recurrence in early-stage primary breast cancer.
EndoPredict, available at Clinical Labs, is the only prognostic test that provides insights into the risk of breast cancer recurrence, the benefits of chemotherapy and the suitability for extended endocrine therapy in women with ER+, HER2- primary breast cancer.
EndoPredict is a second-generation gene expression test for superior prognostic power and is now partially Medicare rebatable.
| Target group check | ||
|---|---|---|
| HER2 negative | Lymph node positive or negative | Tumour size pT1 to pT3 |
| ER positive | Pre- and postmenopausa | Grade 1 to 3 |
-
Why choose EndoPredict?
-
What does EndoPredict report?
-
When to use EndoPredict?
-
How to Order EndoPredict
Request Form Instructions:Please download the EndoPredict request form, fill in the patient details and clinical history, select “EndoPredict,” and indicate whether the patient meets the Medicare Eligibility Criteria.
Ensure that referring clinician details are complete, and if known, provide specimen details. If you would like a copy of the report to be sent to another clinician, please provide the necessary details.
Specimens Required:EndoPredict is performed on FFPE tumour tissue from biopsy or surgical specimens.
Test Cost:The total out-of-pocket cost for Clinical Labs’ EndoPredict test is $1,173.70.
Patients pay $2,275 upfront and can then claim back a partial rebate of $1,101.30 directly from Medicare (eligibility criteria apply).
Patients are required to pay for EndoPredict prior to our laboratory conducting the test. Clinical Labs will contact your patient via SMS or phone call with payment instructions—please advise them to expect this communication in the days following the test request. After making the payment online, Clinical Labs will send your patient a receipt, which they can use to claim the partial Medicare rebate directly from Medicare.
Medicare Eligibility Criteria:Gene expression profiling testing using EndoPredict, for the purpose of profiling gene expression in formalin‑fixed, paraffin‑embedded primary breast cancer tissue from core needle biopsy or surgical tumour sample to estimate the risk of distant recurrence of breast cancer within 10 years, if:
(a) the sample is from a new primary breast cancer, which is suitable for adjuvant chemotherapy; and
(b) the sample has been determined to be oestrogen receptor positive and HER2 negative by IHC and ISH respectively on surgically removed tumour; and
(c) the sample is axillary node negative or positive (up to 3 nodes) with a tumour size of at least 1 cm and no more than 5 cm determined by histopathology on surgically removed tumour; and
(d) the sample has no evidence of distal metastasis; and
(e) pre‑testing of intermediate risk of distant metastases has shown that the tumour is defined by at least one of the following characteristics: (i) histopathological grade 2 or 3; (ii) one to 3 lymph nodes involved in metastatic disease (including micrometastases but not isolated tumour cells); and
(f) the service is not administered for the purpose of altering treatment decisions Applicable once per new primary breast cancer diagnosis for any particular patient.
Turnaround Time:4–5 business days from the sample receipt date.
References
- Filipits M et al. (2011) Clin Cancer Res, 17(18):6012–6020.
- Dubsky P. et al. (2013) Ann Oncol, 24:640–647.
- Buus et al. (2016) J Natl Cancer Inst, 108:11pp.
- Sestak I et al. (2018) JAM Oncology Published online February 15, 2018.
- Sestak I et al. (2018) SABCS 2018.