Precision medicine for breast cancer treatment strategies
By Associate Professor Mirette Saad
Published September 2023
EndoPredict is an in vitro multi-gene prognostic test that provides highly important and clear information for different stages of treatment planning for patients with estrogen receptor-positive (ER+), human epidermal growth factor receptor 2-negative (HER2-) primary breast cancer.
Who to test
Target Group
- Invasive primary breast cancer
- ER-positive
- HER2-negative
- 0-3 pos. lymph nodes
- G1-3
- Size: pT1-3
When to use
EndoPredict is performed on FFPE tumour tissue from biopsy or surgical specimens from patients who have not received systemic endocrine therapy and/or chemotherapy.
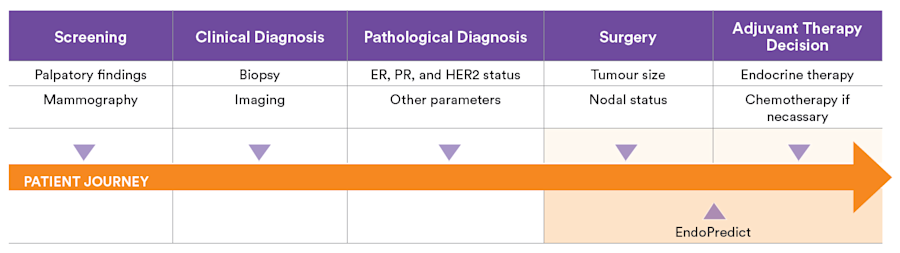
Information is summarised by treatment planning stage
Initial treatment plan
- Risk of recurrence within 0-10 years after diagnosis - Can chemotherapy be avoided?
Each patient’s report will show a graphic curve illustrating their risk of recurrence within years 0-10 after diagnosis. This information helps you to identify patients with a low risk of recurrence who may safely avoid chemotherapy. - Chemotherapy benefit - What is the absolute benefit from chemotherapy?
The second graph in the report illustrates the absolute chemotherapy benefit based on whether the patient receives endocrine therapy alone or endocrine plus chemotherapy. This helps your patient to make a confident decision about chemotherapy treatment.
Long-term treatment planning
- Risk within 5 to 15 years after diagnosis - Can the extension of endocrine therapy be avoided?
The third graph in the report illustrates your patient’s risk of recurrence within years 5-15 after diagnosis.* This information guides treatment decisions regarding endocrine therapy beyond 5 years.
*5-15 year risk is based on treatment with 5 years of endocrine therapy only – no chemotherapy. The result assumes the patient has not experienced recurrence by 5 years.
Comparison with other prognostic tests
When compared to other gene expression tests, EndoPredict was the most prognostic signature for distant recurrence, both in years 0-10 and in years 5-10, in all patients.¹ EndoPredict identified the largest group of women with breast cancer, both in node-negative and node-positive disease:
- at low risk (<10% chance in 10 years) of distant recurrence, who might safely avoid chemotherapy.
- at low risk of late distant recurrence, for whom an extended endocrine therapy might not be justified.
Breast cancer patients and their treating doctors must make complex, highly personalised treatment decisions. Prognostic tools, such as EndoPredict, can play a vital role in determining the type of treatment and prognosis for the patient by assisting with adjuvant therapy decision-making in ER-positive breast cancer. High-quality pathology is a vital part of breast cancer diagnosis and management, and molecular assays such as EndoPredict can provide important additional information to support complex decision-making about the use of chemotherapy in ER-positive breast cancer.
For more detailed information on EndoPredict, please click here.
If you enjoyed this article, subscribe to our electronic Pathology Focus newsletter.
References
- Sestak I. et al. Comparison of the Performance of 6 Prognostic Signatures for Estrogen Receptor- Positive Breast Cancer. A Secondary Analysis of a Randomized Clinical Trial. JAM Oncology Published online February 15, 2018





