QuantiFERON-TB Gold Assay for the Diagnosis of Latent Tuberculosis
Published December 2020
By Associate Professor Owen Harris
Interferon Gamma Release Assays (IGRA)s are in vitro blood tests of the cell-mediated immune system. The QuantiFERON-TB Gold (QFT) assay is an IGRA measuring T-cell release of IFN- γ following stimulation by antigens specific to Mycobacterium tuberculosis complex.
The test
The QFT assay consists of four tubes: the negative-control (nil) tube that measures background IFN, a positive control (mitogen) tube with a non-specific stimulant and two antigen tubes (TB1 and TB2) for diagnosis of latent M. tuberculosis infection.
TB1 contains peptides from the specific Mycobacterium tuberculosis antigens ESAT-6 and CFP-10 designed to elicit an immune response from CD4+ T-helper lymphocytes.
TB2 contains an additional set of peptides targeting a cell-mediated immune response from CD8+ cytotoxic T lymphocytes.
The four tubes are filled with a set amount of whole blood and incubated. During incubation, the antigens stimulate lymphocytes to produce interferon, which is measured by an enzyme immunoassay.
Performance
Since there is no gold standard for latent tuberculosis infection (LTBI), sensitivity and specificity are typically estimated using surrogate reference standards.
Sensitivity is estimated among culture-confirmed TB cases, while specificity is estimated among low-risk individuals with no known TB exposure in low-incidence settings.
Use in children
Due to differences in immune function in young children, (<5 years), QuantiFERON-TB Gold is unreliable and should not be used in this age group.
Interpretation of results
According to the manufacturer, the test is interpreted as positive when either antigen tube result is positive (≥0.35 u/l).
The medical literature, supported by case review experience at Clinical labs, indicates that in a low prevalence population, there is a high rate of false positive (non-specific) results when either TB1, TB2 or both are in the low positive range of 0.35 to 0.70 u/l.
Results in this range need to be carefully correlated with clinical findings and epidemiological risk before making a diagnosis of latent tuberculosis.
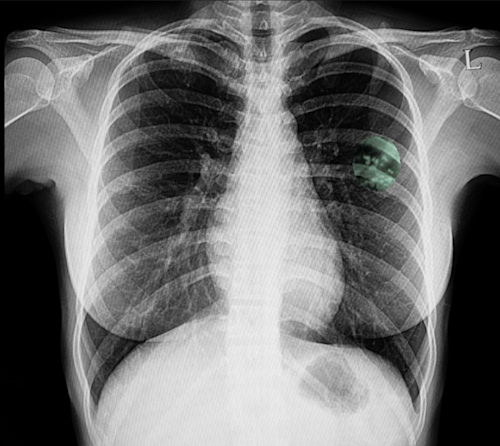
Above image shows calcified nodules in the left upper lobe, consistent with latent tuberculosis.
Indications for treatment of latent tuberculosis
The decision to treat latent TB, to prevent progression to active TB, depends on:
- The pre-test probability, in particular immigrants and expatriates, including health care workers, from countries with a high TB prevalence.
- The risk of progression to active TB: 50% of the lifetime risk of progression occurs within the first 2 years of primary infection. Age-related risks are seen in infants (50%), children (12-25%) and adolescents (10-20%).
- Groups with >5 times risk include patients with HIV infection, children <5 years, patients receiving TNF-α inhibitors, patients with a history of untreated or inadequately treated TB and patients with silicosis, chronic renal failure, leukaemia, lymphoma, or cancer of the upper or lower respiratory tract.
- Groups with 1-5 times risk include patients post gastrectomy, with malnutrition, cigarette smokers, abusers of alcohol and drugs, patients with diabetes mellitus and immunosuppression with agents such as prednisolone (>15 mg daily).
- Efficacy of treatment requires adherence to treatment for up to 9 months. The risk of side effects such as hepatitis (aggravated by age, pre-existing liver disease and alcohol), peripheral neuritis or allergy requires consideration.
A useful reference is Management of tuberculosis: a guide for clinicians, by Denholm JT, Eisen DP, Fox G, McBryde ES, Street A., published by Greenhill Publishing, 2017. ISBN 97890648137979.
If you enjoyed this article, subscribe to our electronic Pathology Focus newsletter.