Navigating the Diagnostic Complexities of Systemic Lupus Erythematosus: Historical perspectives and modern advances
By Associate Professor Louise Smyth
Published May 2025
Systemic lupus erythematosus (SLE), as we know it today, is a Systemic Autoimmune Rheumatic Disorder (SARD) that can affect single or multiple organs and may also present as a disease limited to cutaneous manifestations (DLE). It is from the latter that the term 'lupus' has arisen, having been used for centuries to describe destructive or ulcerating skin lesions of varied aetiology.
Historical perspective
In 1851, Pierre Alphée Cazenave, a French dermatologist, described the facial (butterfly) rash that is archetypal of the disease, naming it lupus érythémateux:
“In some circumstances [lupus] manifests itself at first as a violet rubefaction on this or that part of the face, and mainly on the nose, which at the same time is rather swollen: over many months the colour rises little by little; the surface becomes animated; a small ulcer forms and on top of it, a scab, which then thickens and covers the ulcer, which becomes progressively deeper. Lastly, the skin may get thinner in imperceptible stages and adopt the appearance of a scar, without there being tubercles or ulcers, and without displaying worse injuries than a livid colour and, from time to time, a light and barely perceptible peeling.”
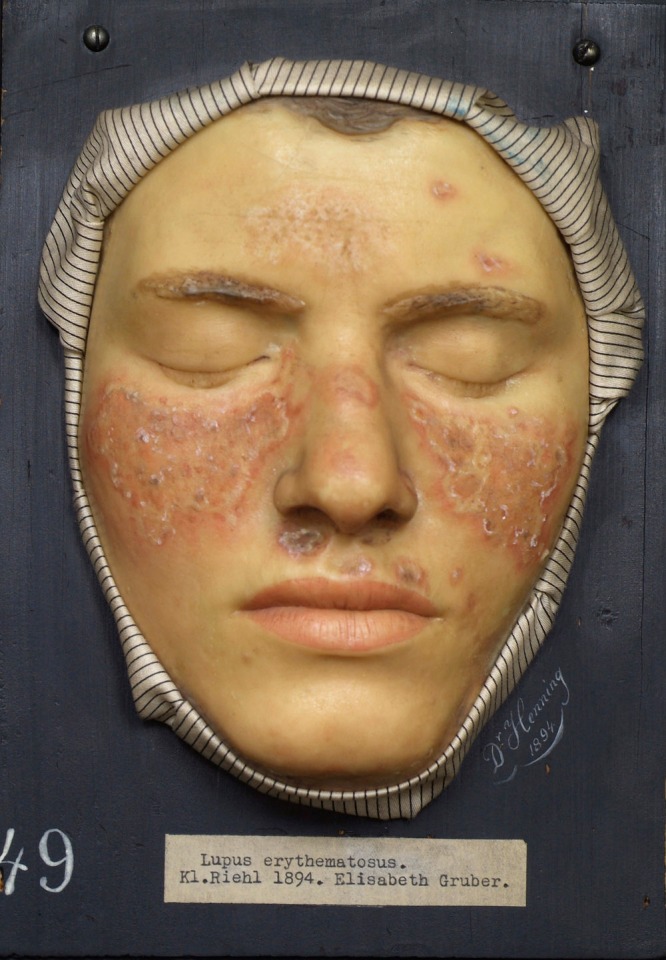
Figure 1. Wax representation of lupus erythematosus (1894) housed in the Federal Pathologic-Anatomical Museum in Vienna. Patzak B. Moulage: "Lupus erythematosus". Dermatol Pract Concept. 2011 Jan 31;1(1):1. doi: 10.5826/dpc. dp0101a01. PMID: 24396710; PMCID: PMC3881073. Copyright ©2011 Patzak.
Moritz Kaposi and Sir William Osler are generally credited with recognising lupus affecting internal organs, in particular, renal and CNS lupus. Descriptions of pulmonary, cardiac and haematological manifestations followed. Keil proposed a single disease that included Discoid Lupus Erythematosus and Systemic Lupus Erythematosus in 1937.
Evolution of diagnosis
As clinical descriptions extended and recognition improved, the incidence of this disorder, recognised in at least one pre-Colombian mummy and possibly linked to Jane Austen, grew. LE cells were described in 1948, followed by an increasing array of autoantibodies, which were generally indicative and sometimes diagnostic. The advent of laboratory tests increased diagnostic capabilities, leading to increased recognition of less serious cases and a dramatic rise in both incidence and prevalence (Felten & al., 2022).
Diagnostic challenges
Lupus remains both a diagnostic and scientific challenge, which sometimes appears to deepen as knowledge expands. The disease we call lupus has multiple presentations within and between patients, may affect one or many organs or tissues, and has a variable course and prognosis. Given this heterogeneous clinical picture, diagnostic assessment will inevitably be equally complex. Since the 1982 American College of Rheumatology (ACR) Revised Criteria for the Classification of Systemic Lupus Erythematosus, circulating autoantibodies have risen in diagnostic importance. Antibodies to native DNA and Sm antigens were added to the criteria, along with the “false-positive serological test for syphilis,” later shown to be anti-cardiolipin antibody.
This set of clinical and laboratory criteria improved diagnostic performance in both sensitivity (83% vs 78%) and specificity (89% vs 87% against a cohort of scleroderma and myositis patients) (Tan & al., 1982). Antinuclear antibody (ANA) by Indirect Immunofluorescence (IIF) was also introduced; however, even then, it was noted to have low specificity, despite being identified in 174 out of 175 SLE patients selected from specialist clinics, demonstrating high sensitivity. Thus, from its earliest inclusion, ANA was proposed as an entry criterion rather than a diagnostic test.
Advances in classification criteria
The ACR criteria were updated in 1997 and remained the reference criteria until the 2012 Systemic Lupus International Collaborating Clinics (SLICC) criteria update. In 2019, the European Alliance of Associations for Rheumatology (EULAR)/ACR classification criteria for SLE were developed. As previously, the need to report homogeneously and consistently in clinical research settings drove the development of classification criteria that have become de facto diagnostic criteria. These latest collaborative criteria have been validated in both adult and paediatric populations (sensitivity 92% and 89%, respectively) and have increased the sensitivity of the 2012 SLICC criteria while protecting the specificity achieved by the 1997 ACR criteria.
Using this standard, positive ANA (at a titre of 1:80) is still defined as an entry criterion, as at this titre, 98% of cases are captured, although its specificity is not sufficient for use as a diagnostic test. ANA at less than 1:80 is seen in other autoimmune disorders, notably in autoimmune hepatitis, where it contributes to the IAIHG simplified scoring system for the detection of autoimmune hepatitis (European Association for the Study of the Liver, 2015).
Delayed diagnosis and disease progression
The clinical diagnosis of lupus is, as stated, complex and is frequently delayed. Rees et al. showed that in the UK, between 1/1/1999 and 31/12/2012, patients who were eventually diagnosed with lupus presented to their general practitioners a median of 9.2 times per annum during the 5 years preceding diagnosis for symptoms associated with lupus, compared to 3.8 times per annum for case-based controls (Rees, 2017). Additionally, it has been known for several years that some autoantibodies, including anti-dsDNA, can be identified in the serum of individuals who are clinically well but who would later be diagnosed with lupus (Arbuckle, et al., 2001).
As with anti-CCP antibody in rheumatoid arthritis, the concentration of antibody increases proximate to the development of clinical disease. Perhaps unsurprisingly, in a disorder that presents with a range of severity, milder and more frequent cases initially present to family practitioners, while cases that present directly to specialists with greater experience of this nebulous disease tend to be more severe. Nikopolous et al. demonstrated that, for Caucasian patients with a disease duration of at least a year, approximately 55% had a mild phenotype at diagnosis, but nearly half of those progressed to more severe disease. After three years, there was a more than 20-fold increased risk of accrued irreversible tissue damage (Nikolopoulos, et al., 2020). In their study, high-risk patients were men, those with positive anti-dsDNA or those with neuropsychiatric disease.
Ethnic and age-related variation
Epidemiological differences in disease frequency and severity have been well documented. Pons-Estel et al. (2010) summarised that lupus is 2-4 times more frequent and severe in non-white populations. Although lupus most commonly affects women of childbearing age or later, it tends to be more severe in men, paediatric patients (<17 years at diagnosis) and late-onset patients (> 50 years at diagnosis).
EULAR/ACR classification criteria
The EULAR/ACR criteria were published in the Annals of the Rheumatic Diseases (Aringer, 2019) and a guide to the relevance of common ANA patterns was published by Lazar in 2023 (see below). All laboratory investigations listed in the EULAR/ACR criteria are available at Clinical Labs.
Table 1. Guide to the relevance of common ANA patterns
| Antinuclear antibody pattern | Associated antigen targets | Clinical disease correlate |
|---|---|---|
| Homogeneous | dsDNA, histones, chromatin | SLE, drug-induced SLE, JIA, chronic autoimmune hepatitis |
| Dense fine speckled | DFS70 | Healthy and other non-SARD conditions |
| Centromere | CENP-B (centromere) | Limited cutaneous SSc, PBC |
| Fine speckled | SS-A/Ro, SS-B/La, Mi-2, TIF1y, Ku | SjS, SLE, SCLE, neonatal lupus erythematosus, congenital heart block, DM, SSc, SSc-AIM overlap |
| Coarse speckled | Sm, RNP, U1RNP, RNA-polymerase III | SLE, SSc, MCTD, SSc-AIM overlap, UCTD |
| Nucleolar | Th/To, PM/Scl, U3RNP, RNA-polymerase I | SSc, SSc-AIM overlap, Raynaud’s, SjS,cancer |
Abbreviations: AIM, autoimmune myositis; DM, dermatomyositis; JIA, juvenile idiopathic arthritis;
MCTD, mixed connective tissue disease; PBC, primary biliary cirrhosis; SARD, systemic autoimmune
rheumatic disease; SCLE, subacute cutaneous lupus erythematosus; SLE, systemic lupus erythematosus;
SjS, Sjogren’s syndrome; SSc, scleroderma; UCTD, undifferentiated connective tissue disease.
Copyright © 2023 by the author(s) (Lazar & J., 2023)
If you enjoyed this article, subscribe to our electronic Pathology Focus newsletter.
Subscribe Today!How to Order Laboratory Investigations for Systematic Lupus Erythematosus
The Clinical Labs general pathology request form can be used to request the following laboratory investigations listed in the above EULAR/ACR criteria:
Antinuclear antibodies (ANA): for initial assessment; followed by SLE-specific antibodies and serum complement when positive (anti-dsDNA antibody, ENA) or as directed in ANA report.
Antiphospholipid antibodies: (Anti-cardiolipin antibodies, Anti-ß2GP1 antibodies, Lupus anticoagulant), especially if thromboembolic disorder or pregnancy loss.
Clinical history is important for the laboratory interpretation of the results.
Bulk-billed, subject to Medicare eligibility criteria.
References
Arbuckle, M. R., James, J. A., Kohlase, K. F., Rubertone, M. V., Dennis, G. J., & Harley, J. B. (2001). Development of Anti-dsDNA Autoantibodies Prior to Clinical Diagnosis of Systemic Lupus Erythematosus. Scandinavian Journal of Immunology, 54, 211-219. Retrieved from https://doi.org/10.1046/j.1365-3083.2001.00959.x
Aringer, M. e. (2019). 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Annals of the rheumatic diseases, 78(9), 1151–1159. doi:https://doi.org/10.1136/annrheumdis-2018-214819
European Association for the Study of the Liver. (2015). EASL Clinical Practice Guidelines: Autoimmune hepatitis. Journal of Hepatology 2015; 63:971-1004, 63(4), 971-1004. doi:10.1016/j.jhep.2015.06.030.
Felten, R., & al., e. (2022). The History of Lupus Throughout the Ages. Journal of the American Academy of Dermatology, 87(6), 1361–1369. Retrieved from https://doi.org/10.1016/j.jaad.2020.04.150.
Lazar, S., & J., M. K. (2023). Systemic Lupus Erythematosus: New Diagnostic and Therapeutic Approaches. Annual Review of Medicine, 339-352. doi:https://doi.org/10.1146/annurev-med-043021-032611
Nikolopoulos , D. S., Kostopoulou, M., Pieta , A., Flouda , S., Chavatza, K., Banos, A., . . . Fanouriakis, A. (2020). Transition to severe phenotype in systemic lupus erythematosus initially presenting with non-severe disease: implications for the management of early disease:. Lupus Science & Medicine 2020;7:e000394.,7:e000394.
Pons-Estel , G. J., Alarcón , G. S., Scofield, L., & Reinlib, L. (2010). Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum., 39(4), 257-268. doi:10.1016/j.semarthrit.2008.10.007. Epub 2009 Jan 10. PMID: 19136
Rees, F. e. (2017). Early Clinical Features in Systemic Lupus Erythematosus: Can They Be Used to Achieve Earlier Diagnosis? A Risk Prediction Model. Arthritis Care & Research, 69(6), Arthritis Care & Research,. Retrieved from https://acrjournals.onlinelibrary.wiley.com/doi/epdf/10.1002/acr.23021
Tan, E., & al., e. (1982, 11). The 1982 Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis and Rheumatism, 1271-1277. Retrieved from https://onlinelibrary.wiley.com/doi/epdf/10.1002/art.1780251101