Australian Clinical Labs and Geneseq Biosciences announce the launch of the new Melaseq™ Melanoma Genomic Testing
Published December 2024
Australia is the skin cancer capital of the world, with over 1.5 million biopsies performed a year resulting in approximately 17,000 invasive melanomas diagnosed. Tragically, each year, approximately 1,400 people die from this form of cancer, despite early detection often being curative and promising advances in treatment such as immunotherapy. The best chance of surviving melanoma remains prevention, early detection, accurate diagnosis and effective treatment.
Melaseq is an Australian-developed and owned test that enables doctors to more accurately diagnose melanoma in its earlier stages. Using genomic technology to define the genetic fingerprint of invasive melanoma, researchers at Geneseq Biosciences, led by Dr Ryan Van Laar, developed a test that can detect the earliest signs of the disease.
Australian Clinical Labs has partnered with and invested in Geneseq Biosciences’ technology to make this innovative, world-first, technology available to all patients across Australia. Australian Clinical Labs' CEO Melinda McGrath says, "We are proud to have partnered with Geneseq Biosciences in bringing this groundbreaking test to clinicians and patients in Australia. This innovation has the potential to transform patient outcomes worldwide, and we are excited to be pioneers in implementing new technologies to help lower the burden of this highly metastatic form of cancer. The Melaseq test exemplifies our commitment to advancing diagnostic technologies that enhance patient care."
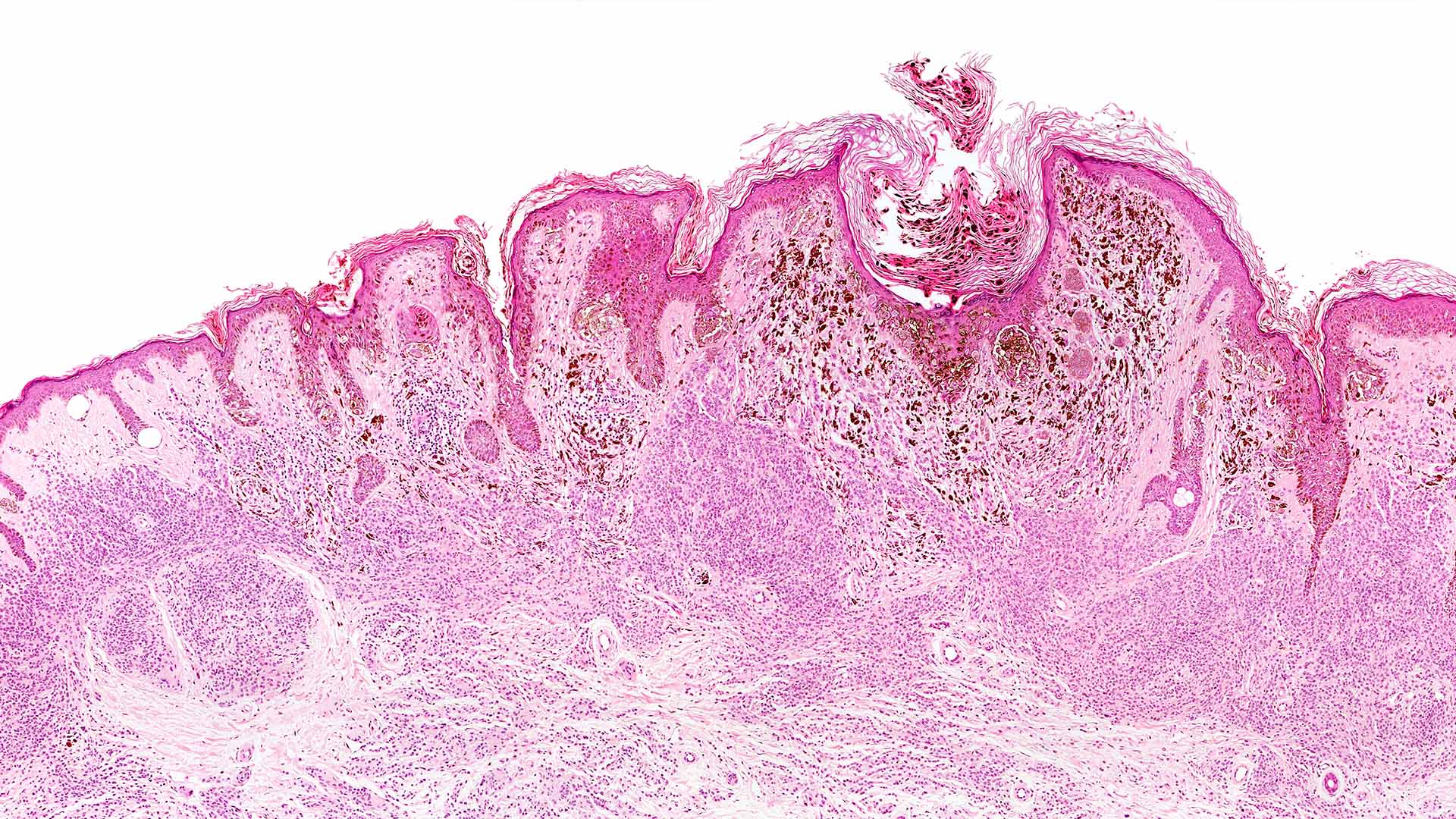
The newly approved test analyses the activity of genomic sequences called microRNAs, which cells use to control which genes are switched on or off. These types of RNA are present in all cells and even in the blood stream, however, their activity begins to change as many cancers, including melanoma begin to develop and invade. The Melaseq (Solid Tissue) test can be performed on the cells biopsied by a clinician during a skin examination. The test generates a personalised Melanoma Genomic Score, which informs the clinician of the level of malignancy present at a genomic level, helping them determine the appropriate care path for each patient.
"This is a significant milestone for both our organisations and the Australian healthcare community," says Dr. Ryan Van Laar, Ph.D., Founder of Geneseq Biosciences. "This technology leverages the sensitivity and specificity of genomics, coupled with the latest understanding of melanoma biology. We're confident this test will improve diagnostic accuracy and increase access to the latest in cancer genomics right across the country."
Current melanoma diagnosis relies on a clinician or pathologist’s visual examination of skin tissue and cell structures. Melaseq (Solid Tissue) provides a deeper assessment of malignancy, by looking inside skin cells at the genetic level, using advanced genetic barcoding and data analytics. The result is an objective and reproducible assessment of cancer status, demonstrating over 95% accuracy in multiple clinical studies. The test complements traditional diagnostic procedures by providing highly personalised and previously unavailable information.
The Melaseq Solid Tissue test is performed on the same small piece of skin a doctor removes for diagnostic assessment, which happens more than 1.5 million times in Australia annually—eliminating the need for patient additional clinic visits or additional invasive diagnostic procedures. Work is under way on the blood-based (“liquid biopsy”) version of the same test, which will complement skin exams and other screening procedures—subject to regulatory approval within the coming months.
Both applications of the Melaseq test have the potential to increase all Australian’s access to the latest advances in melanoma early detection technology. The test may be ordered as part of routine healthcare check-ups or recommended by clinicians based on each patient’s melanoma risk profile.